 Mesomery
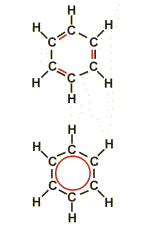
Mesomery
The chemical formulas for representing covalent bonds are based on the simplified assumption that the electrons of a given bond can be treated isolated from the other electrons of the molecule. In the case of conjugated double bonds, this simplification is no longer possible. Such bonds are better described by a model combining the electrons of all conjugated bonds and distributing them over the participating carbon atoms (formula below). This phenomenon is called mesomery. Typical examples for mesomery are aromates and conjugated double bonds.