 Aromates
Aromates
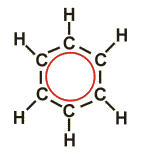
(Mostly) circular molecules containing 1, 3, 5, 7 or more conjugated double bonds. Due to the laws of quantum mechanics, conjugation produces especially stable systems for the numbers mentioned. Conjugated double bonds are better regarded as a unit of electrons stretching over the participating carbon atoms than as a group of individual double bonds.
The compound shown (benzene) is made up of six carbon atoms and contains three double bonds. In order to symbolize the distribution of the respective electrons over the complete ring, the symbols for the individual double bonds have been replaced by the red circle.