 Carbonic acids
Carbonic acids
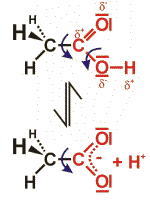
The carbon atom of carbonic acids is connected to an oxygen atom (via a double bond) and to a hydroxy-(OH) group (via a single covalent bond).
As an example, the structural formula of a carbonic acid consisting of two carbon atoms is given (acetic acid). Due to the influence of the oxygen atom, the carbon and the hydrogen atoms are partially charged positively – the proton can easily be liberated. (Causing the acidic nature of the compound.) After liberation of the proton, the negative charge of the molecule is stabilized by mesomeric dislocation over the two oxygen atoms.
Due to the double bond, both oxygen and carbon atoms lie within one common plain. The covalent bond between the carbon atoms can rotate freely. Accordingly, there are many possible structures; the structure shown gives only one of these possibilities.
Carbonic acids are formed by oxidation of alcohols or aldehydes.