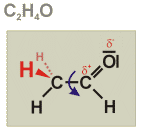
 Structural formula
Structural formula
The structural formula of a given molecule represents the spatial arrangement of its atoms and the way these atoms are connected to each other.
The structural formula of acetaldehyde is shown as an example. Each dash represents a pair of electrons from the outer layer of the electronic sheath. Dashes between individual atoms correspond to covalent bonds, dashes close to single atoms correspond to free pairs of electrons. All atoms marked in black are lying within one common plain (marked in grey). Only the hydrogen atoms marked in red are outside of this plain. The blue arrow marks the fact that the partner of the respective bond can rotate without restriction against each other. The red symbols at the aldehyde group give the partial charges of the respective atoms.