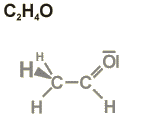
 Empirical formula
Empirical formula
The empirical formula gives the number of the various atoms a molecule is composed of. It does not contain information about the way these atoms are linked to each other (see structural formula).
The empirical formula for acetaldehyde given as an example tells that this molecule contains two carbon-, four hydrogen- and one oxygen atom.