 Single bonds
Single bonds
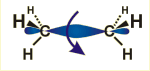
The graphical model shows the distribution of electrons in the case of a single bond. (Only the bond between the two carbon atoms is depicted. The other groups bound to the carbon atoms (hydrogen atoms in this case) are given as symbols.)
Both partners of a single bond can rotate freely against each other. Accordingly, there are many possible structures; the structure shown gives only one of these possibilities. The other partners bound to carbon atoms in question are situated in the corners of a tetraeder (separated by 109 degrees each).