 Amines
Amines
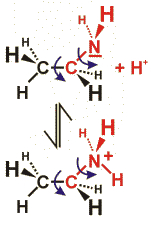
In the case of amines, a nitrogen atom is connected to one or several (up to four) carbon atoms. Amines are bases, because they easily bind a positively charged hydrogen atom (proton).
The example gives the structural formula of an amine containing two carbon atoms (ethanolamine). By taking up a proton, the molecule becomes positively charged. The covalent bonds connecting the carbon and nitrogen atoms can rotate freely. Accordingly, there are many possible structures; the structure shown gives only one of these possibilities.