 Carbohydrates
Carbohydrates
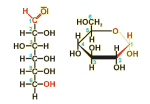
In nature, carbohydrates are used for the transfer of energy (like glucose depicted in the figure) and as structural elements (for example cellulose).
The name and empirical formula of carbohydrates (C6(H2O)6 for glucose, for example) suggest, they might be formed by the combination of carbon and water. In reality, however, they are partially oxidized hydrocarbons, containing a keto- or aldehyde group. Because of these reactive groups, many carbohydrates are forming rings which can be combined with each other leading to larger molecules. Depending on the number of molecules combined, the compounds are called monosaccharides (only one molecule), oligosaccharides (several combined molecules, e.g. disaccharides, containing two or trisaccharides containing three molecules) and polysaccharides (a large number of combined molecules, as in the case of cellulose or starch from plants and chitin from fungi or arthropods).