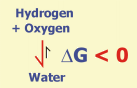 Chemical equilibrium
Chemical equilibrium
The concentrations of products and reactants for a given chemical reaction in the state of chemical equilibrium can be calculated from the amount of Gibbs Free Energy (G) for this reaction.
The example shows the reaction of hydrogen and oxygen to water. Gibbs Free Energy is highly negative for this reaction. Therefore, high amounts of water and small amounts of hydrogen and oxygen are present, when this reaction has reached chemical equilibrium.
The reaction between hydrogen and oxygen can be easily started and quickly reaches equilibrium. Other chemical reactions, however, need a more or less large amount of activation energy to be started. The reaction of wood with oxygen, (the burning of wood) for example is characterized by a large amount of activation energy. Accordingly, wood is stable in oxygen containing air, although the chemical equilibrium is similar when compared to the reaction of hydrogen and oxygen.