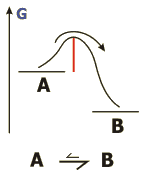
 Activation energy
Activation energy
The amount of energy, that has to be surpassed in order to reach chemical equilibrium.
The activation energy determines how long it takes until the chemical equilibrium of a given reaction is reached. Catalysts reduce this amount and therefore shorten this time.