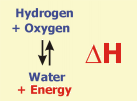
 Enthalpy (H)
Enthalpy (H)
Enthalpy refers to the amount of energy that is liberated (negative enthalpy) or consumed (positive enthalpy) in a chemical reaction.
The reaction of hydrogen and oxygen to water, which liberates a large amount of energy, is given as an example. After mixing the two compounds, the explosive reaction is started by a very small amount of energy (activation energy). Under special conditions, however, it is possible to revert the reaction and to produce hydrogen and oxygen from water (and energy).
In general, chemical reactions are not only determined by the amount of energy liberated, which is dominating in the case of hydrogen and oxygen, but also by changes regarding the molecular disorder (entropy). The combination of both factors results in Gibbs Free Energy and allows to predict the concentrations of reacting compounds and products in the state of chemical equilibrium.