 Isotopes
Isotopes
Atoms with an identical number of protons but differing numbers of neutrons.
Because neutrons donīt influence the electronic sheath of atoms, they donīt influence the chemical characteristics of atoms as well. Accordingly, all isotopes belong to the same chemical element. All isotopes with one proton, for example, belong to the chemical element hydrogen.
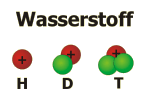
The mass differences of the various isotopes are particularly large in the case of hydrogen; therefore these isotopes have their own names: Deuterium (D) with one neutron (mass number 2), tritium (T) with two neutrons (mass number 3).
Atomic nuclei are only stable, when the numbers of protons and neutrons are not too large and when these particles are present in a certain ratio. Isotopes, which do not fullfill these criteria (as for example tritium), are disintegrating by emitting energetic radiation (radioactivity). Most chemical elements refer to a small number of stable isotopes, which are existing in the same ratio to each other under most conditions. Hydrogen, for example, contains 0,015 percent deuterium and very small traces of radioactive tritium. 99,985 percent refer to the isotope with no neutrons.