 Mass number
Mass number
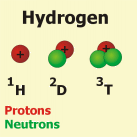
The mass number of a given atom refers to the sum of its protons and neutrons.
This number is prefixed to the elemental symbol. (The example shows the isotopes of the chemical element hydrogen).
Because the neutrons donīt influence the electronic sheath, there is no connection between the mass number and the chemical characteristics of a given atom. Atoms differing only in the number of neutrons, but not in the number of protons (isotopes) are therefore belonging to the same chemical element.