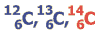 Radiocarbon dating
Radiocarbon dating
Method for the determination of the age of biological materials.
Atmospheric carbon dioxide contains small amounts of the radioactive carbon isotope 14 (14C). This unstable isotope is formed continously by cosmic radiation in the atmosphere. Due to radioactive decay of the element, its amount is decreasing continously after integration into biological structures by plant photosynthesis. Measuring the remaining amout of radioactive carbon allows dating of the material back to about 50 000 years.