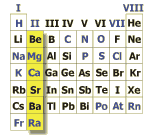
 Alkaline earth metals
Alkaline earth metals
Named after arabic "al kalja" for soda ash (sodium carbonate) from the ashes of waterplants. Hydroxides of alkaline earth metals are strong bases like hydroxides of alkaline metals, but they are dissolving less easily in water.
The elements beryllium, calcium, magnesium, strontium und barium are constituting the alkaline earth metals. They are easily liberating two electrons, forming twofold positively charged ions. When the electrons are transferred to water (in a usually violent reaction) molecular hydrogen (H2) and hydroxid ions are formed.