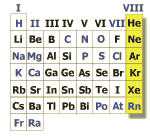
 Noble gases
Noble gases
Helium, neon, krypton, argon and xenon are belonging to the noble gases.
The electronic sheath of noble gases is constituted in an ideal way. Therefore respective atoms have neither the tendency to take up, nor to liberate electrons or to form covalent bonds. They are not reacting with other atoms, forming inert atomic gases.