The chapter described in the following post is part of a very graphical powerpoint presentation on soil microbiology. This presentation is freely available at the Helmholtz-Centre for Environmental Research (UFZ) in Leipzig, Germany.
Chapter 1 of the presentation soil microbiology covers the physicochemical basis – soil itself.
Soil chemistry is depending on the distribution of fluids and gases in soil,
- Distribution of solid, liquid and gaseous phases in soil
which, in turn depends on the distribution of soil pores.
- Soil pores
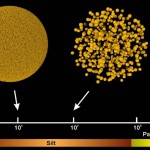
The distribution of soil pores, in turn, depends on the size distribution of soil particles and on aggregation of these particles. Here particle populations of identical volume, but with a tenfold difference in diameter are shown, to demonstrate the connection between particle size and pore size.
- Particles and pores
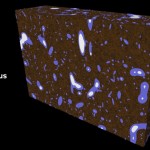
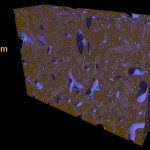
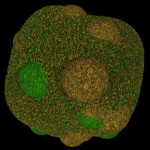
Soil particles are combined into soil aggregates and, ideally, those aggregates contain organic material (based on carbon and given in green color) and inorganic material (based mostly on silicon and given in brownish color).
- Soil aggregate
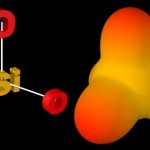
Silicic acid (one major component of inorganic soil material) is formed by the complete oxidation of silicon and is a very versatile building block of a large number of minerals.
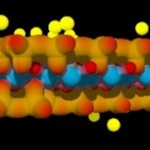
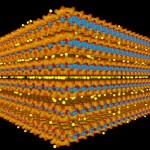
The most important ones of such minerals (in terms of being able to serve as nutrient reservoirs for plant life) are three-layered clay minerals. Individual layers consist of two silicate layers (brownish-red), one central layer of alumina (blue color) and positive ions (yellow color) attached to the outer surfaces of the silicate layers.
- Three-layered clay mineral
Such minerals may bind large numbers of ions like ammonia or potassium (given in yellow color), which are important for plant nutrition.
Further reading:
What Soil Aggregates Are and How its Stability Affects Soil Erosion
Pingback: Christmas – How to Dispose of a Tree - Fun Holiday Store